Table Of Contents:
The Solution: A Mechanistic Digital Twin for Your End-to-End (E2E) Process
Case Study: End-to-End System Modelling for a Reactive Crystallization Drug Substance Process
Conclusion
References
A common challenge in pharmaceutical development is scaling up complex processes. After promising lab results, moving to pilot and manufacturing scales often leads to Critical Quality Attribute (CQA) variability, costly failures, and delays [1]. This challenge is magnified when we consider the complexity of a series of interconnected unit operations required to manufacture products. What happens in an early step, like reactive crystallization, can have unforeseen, non-linear effects on downstream steps like filtration and drying.
Traditionally, the industry has relied on extensive experimental trials to characterize systems, but this is often insufficient to manage this complexity. The experiment-heavy approach has several limitations [2], [3]:
High Cost and Inefficiency: It requires a large number of physical experiments to explore process factors, which is both time-consuming and expensive.
Limited Scope: Current methods restrict capability for exploring the complex, multifactorial interactions between process parameters and across different process steps. This hinders the ability to identify the most influential factors.
Reactive Insights: The insights from experiments are retrospective, emerging only after the experiments are performed. This reactive approach can lead to unexpected additional work and delays in decision-making.
Scale-Up Risk: As a result, translating the process from lab to commercial production can be unreliable. The scaled-up process is often not as robust or optimized as it could be.
The Solution: A Mechanistic Digital Twin for Your End-to-End (E2E) Process
To address these limitations, a more predictive, knowledge-driven approach is required. The solution is E2E System Modeling, which creates a holistic digital twin. These tools complement experimental work, facilitating accelerated development and mitigating risks associated with scale-up. This model is defined by two key concepts:
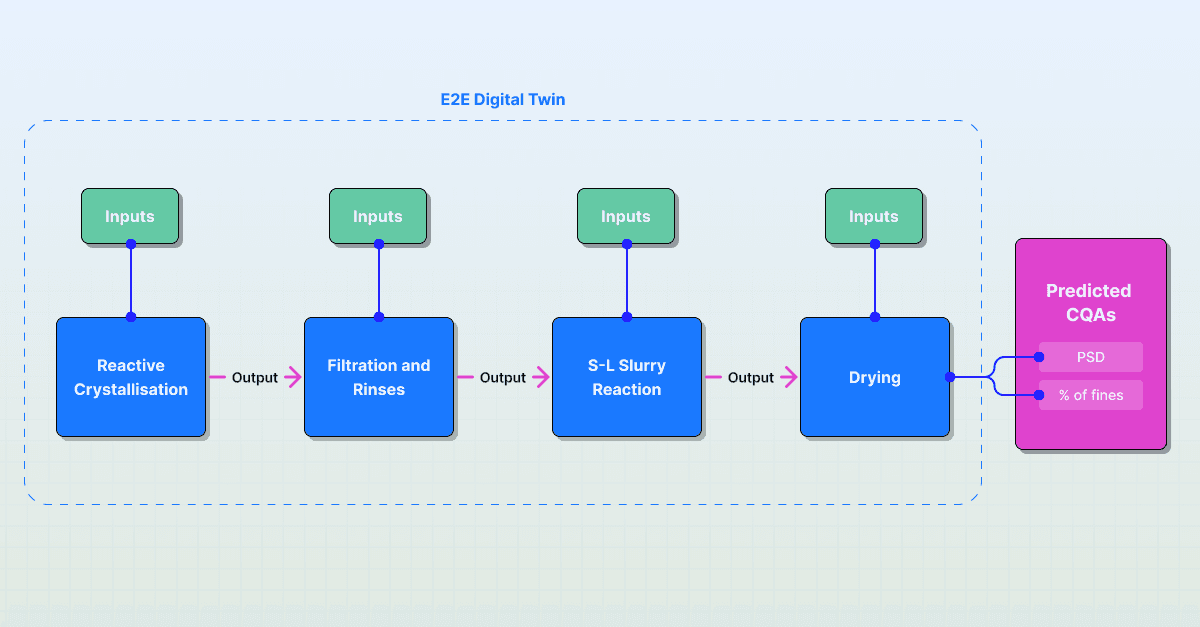
Connected Unit Operations: The model involves all key unit operations. The framework is designed so that the outputs from one step are used as the inputs for the next. For example, the Particle Size Distribution predicted from the reactive crystallization step serves as the input for the filtration model. This addresses the limited scope problem by modeling the process as one integrated system.
Mechanistic Approach: Unlike Design of Experiments (DoE), which is limited to statistical correlations, the model is built on first-principles physics and chemistry. This enables the capture of the complex, non-linear behaviors and multifactorial interactions that DoE misses.
The primary benefit of this model-informed approach is the ability to run in silico (computer-based) analyses, which directly counters the high cost and reactive nature of physical experiments. Such models require far fewer calibration data points than a traditional DoE.
Case Study: End-to-End System Modelling for a Reactive Crystallization Drug Substance Process
The Challenge
A project team at a top 5 pharma company was developing a complex drug substance process involving reactive crystallization. The final CQAs, such as the percentage of fines, were highly sensitive to initial process conditions. Initial small-scale studies showed that certain product attributes had variability during scale-up from lab to commercial operations. The non-linear effects were a key challenge, and a traditional DoE path was deemed too high-risk, costly, and slow.
The Model-Informed Approach
An end-to-end system model was built to simulate the key manufacturing stages: reactive crystallization, filtration and rinses, S-L slurry reaction, and drying. The team used this model to run Virtual DoEs. This allowed them to test how initial reactor parameters would affect both intermediate CQAs and the final product attributes.
The model identified the true critical drivers across the entire process chain. For example, Global Sensitivity Analysis (GSA) revealed that while the drying step was key to CQA variability, some variability actually originated in the reactor step and was exacerbated during drying. This mechanistic insight allowed the team to understand why and when CQAs were deviating.
The Outcome
Financial: $2.2 million in savings in API and direct input into a commercial decision worth hundreds of millions.
Speed & Efficiency: The team was able to reduce the number of lab-scale reaction runs from 128 to 50, accelerating the project timeline by over 3 months.
Sustainability: The reduction in experimental work and solvent use resulted in 115 tonnes of CO2e savings per year.
The team could virtually design a robust process. The model provided a clear map of the safe operating window for manufacturing, which de-risked tech transfer and scale-up.
Conclusion
For pharma manufacturers, the adoption of end-to-end process modeling represents a strategic shift to proactive, predictive process development. By grounding development in the fundamental physics of the entire process chain, companies can build deep, holistic process understanding, identify critical drivers across unit operations, reduce their reliance on costly physical experimentation, and significantly de-risk tech transfer and scale-up. The result is a more robust process and a faster path to market for high-quality medicines.
At Polymodels Hub, it’s our mission to help pharma companies harness data and models in apps and workflows to accelerate drug development.
Book a demo to see how an E2E model can accelerate your R&D pipelines.
References
F. Destro, P. K. Inguva, P. Srisuma, and R. D. Braatz, ‘Advanced methodologies for model-based optimization and control of pharmaceutical processes’, Curr. Opin. Chem. Eng., vol. 45, p. 101035, Sept. 2024, doi: 10.1016/j.coche.2024.101035.
ISPE, ‘In-Silico Data-Driven Mechanistic Model–Assisted Process Validation | Pharmaceutical Engineering’. Accessed: Dec. 05, 2025. [Online]. Available: https://ispe.org/pharmaceutical-engineering/may-june-2024/silico-data-driven-mechanistic-model-assisted-process
N. Yazdanpanah, C. N. Cruz, and T. F. O’Connor, ‘Multiscale modeling of a tubular reactor for flow chemistry and continuous manufacturing’, Comput. Chem. Eng., vol. 129, p. 106510, Oct. 2019, doi: 10.1016/j.compchemeng.2019.06.035.