Table Of Contents:
What if your most valuable scientists are spending more time on data entry than on analysis?
The Challenge: A 300-Hour Manual Bottleneck
The Solution: Project ARA
The Impact: More Than Speed - Enabling Deeper Insights and Trust
Conclusion
What if your most valuable scientists are spending more time on data entry than on analysis?
A top 10 pharma client faced this exact problem: a 300-hour annual bottleneck translating unstructured, multilingual lab notes into Process Flow Diagrams and Technical Risk Assessments. This case study details the solution we built to accelerate this workflow, moving their team from rigid spreadsheets to a dynamic, AI-driven system.
Key Results at a Glance
Reduction in Process Flow Diagram and Technical Risk Assessment workflow time
Centralized Knowledge by creating a standardized, reusable library for risk assessments
Eliminated rigid Excel-based documentation
The Challenge: A 300-Hour Manual Bottleneck
In preclinical process development, bridging the gap between exploratory lab work and a future GMP tech transfer can be challenging. It’s not easy to go from unstructured lab notes into an initial Process Flow Diagram and Technical Risk Assessment.
A top 10 pharma client found themselves spending over 300 hours per year on this task. Their process involved manually translating electronic lab notes into a Process Flow Diagram and Technical Risk Assessment, creating two major bottlenecks:
An unscalable approach using Excel: Engineers and scientists had to read through unstructured Electronic Lab Notebooks (ELNs), manually identify manufacturing steps, and then track this entire process in Excel. This tool, not designed for dynamic process flows, proved rigid; inserting a new process step, for example, could disrupt the entire document.
Lack of standardization and reusability: The client's process depended on individual knowledge. Risk assessment questions had to be re-created from scratch for each new project, duplicating effort.
This high-effort process meant that the majority of valuable engineering time was spent on manual documentation rather than on critical risk analysis.
The Solution: Project ARA
To address these challenges and streamline the creation of Process Flow Diagrams and Technical Risk Assessments, we developed the Automated Risk Assessment (ARA) tool. The ARA tool transforms the client's unstructured ELN documents and batch records into structured, dynamic Process Flow Diagrams and Technical Risk Assessments.
This project was particularly difficult because the client's ELN documents:
Contained scientist notes without a standardized format.
Included multiple entries corresponding to a single manufacturing step.
Were written in different (and sometimes even multiple) languages.
This combination of unstructured, multilingual data is well-suited for Large Language Models (LLMs) [1]. Where traditional, rules-based methods would struggle, an LLM can use context from its training data to interpret varied formats and languages.
The ARA system is designed to provide speed without sacrificing scientific control. We prevent LLM hallucinations by fundamentally constraining its role, creating a multi-step, iterative human-in-the-loop workflow.
The workflow is broken into distinct, verifiable phases:
Extraction to an Intermediate Object: First, the LLM takes the raw, unstructured ELN document and extracts a clarified, sequential experimental procedure.
Human Fidelity Check: Before any further transformation, an engineer reviews, edits, and verifies the fidelity of this extracted procedure. This ensures the source data for the next step is 100% accurate and human-approved.
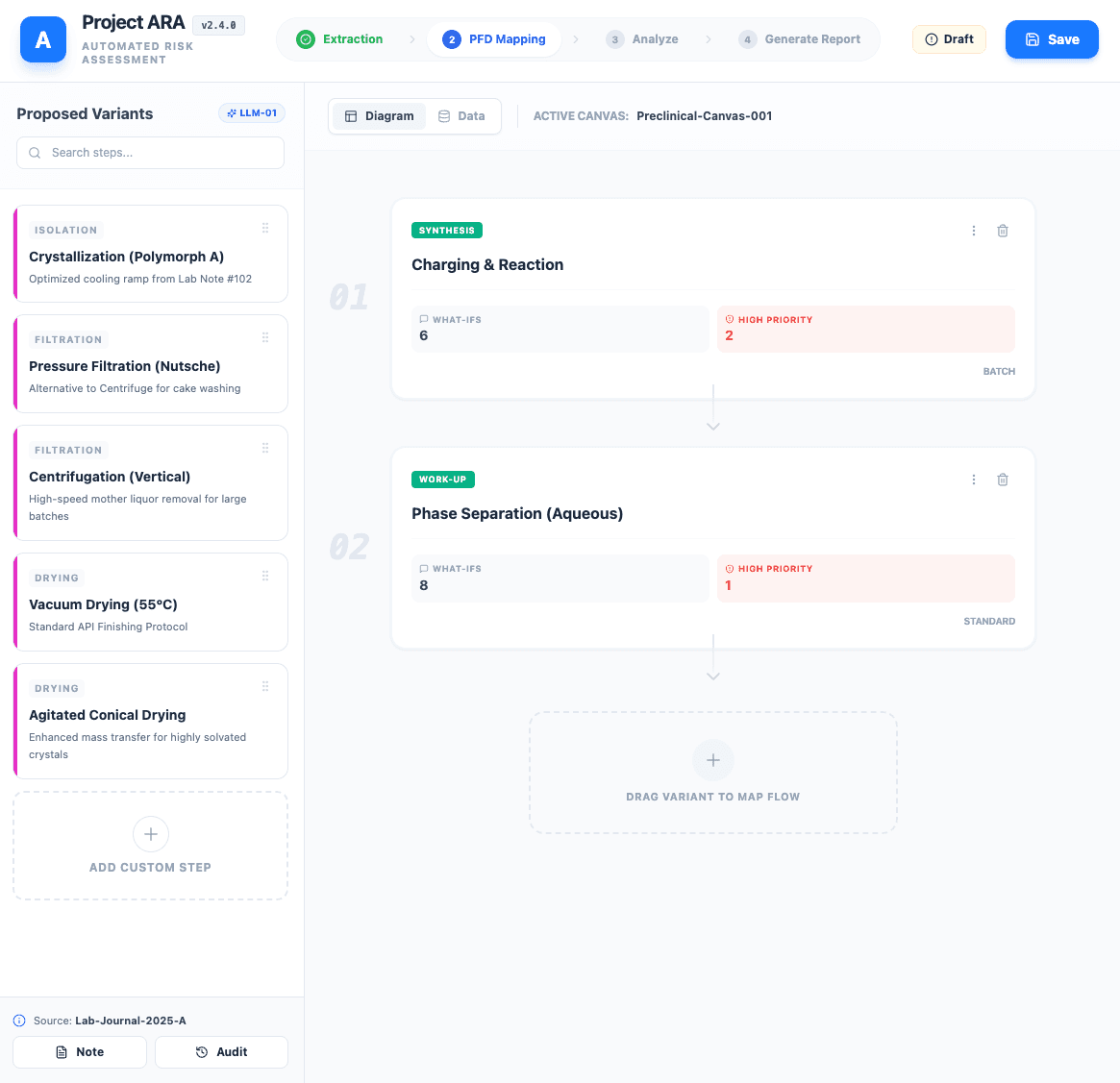
Iterative Process Flow Diagram Mapping: Once the full experimental procedure is verified, the system begins mapping it to the Process Flow Diagram. This is an iterative process, controlled by the engineer:
Variant Generation: The system proposes 3 variants of the first few manufacturing steps. This mapping is constrained to a controlled vocabulary - a pre-defined database of user-approved steps - to prevent hallucination.
Human-in-the-Loop Selection: The user reviews the 3 options, selects the best variant, and makes any necessary edits.
Iterate to Closure: Once the first few steps are selected and verified, the system generates variants for the next steps. This "generate-verify-repeat" cycle continues until the entire process flow is mapped and approved.
Standardized Technical Risk Assessment Seeding: With the Process Flow Diagram flow finalized, ARA automatically seeds the risk assessment. The system pulls from a central bank of pre-defined "what-if" questions for each process step, providing a standardized foundation for the Technical Risk Assessment. Engineers can then select, prioritize, and add custom questions.
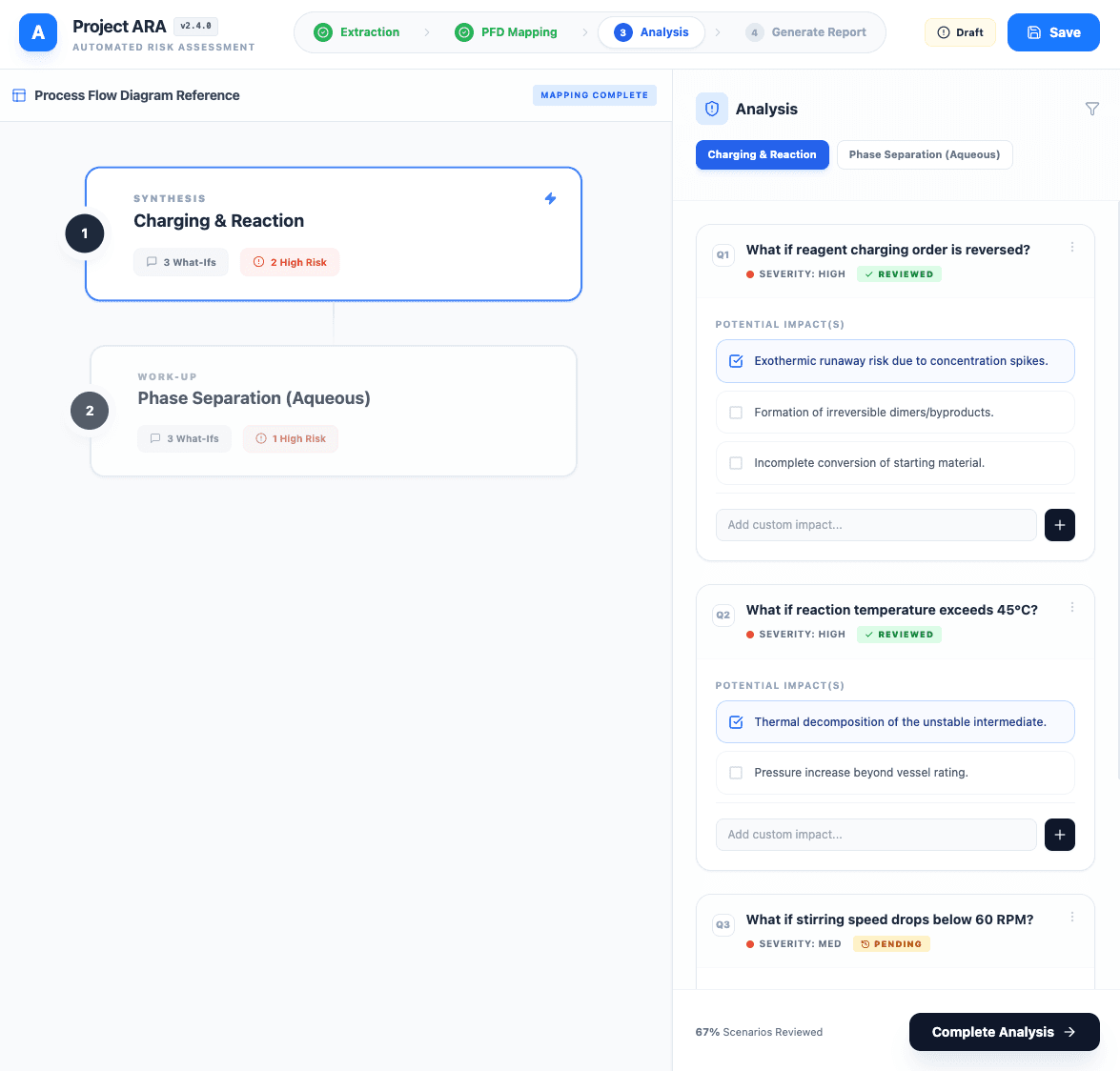
The Impact: More Than Speed - Enabling Deeper Insights and Trust
The ARA tool provides more than just a speed increase; it changes the client's relationship with their own data and processes. By significantly decreasing the effort of documentation, the tool reduced friction and error rates associated with manual data entry.
A Shift from Documentation to Analysis: With the documentation burden lifted, the client's teams can now explore multiple manufacturing routes and focus their expertise on high-value risk analysis, rather than on manual data entry.
Building Trusted, Institutional Knowledge: The iterative, human-in-the-loop workflow was critical. Because engineers verify each step - from the initial procedure extraction to the final Process Flow Diagram - the resulting knowledge base is fully vetted and trusted. This builds a central, living library of institutional knowledge, not just a static report. It ensures the team builds a deep, shared understanding of the process.
Conclusion
For pharma manufacturers, the transition to AI-enabled workflows like Project ARA represents more than just a productivity gain; it is a strategic shift to expert-led, data-driven development. By removing the 300-hour burden of manual data entry, scientists are freed to do what they do best: analyze risks, optimize processes, and accelerate the delivery of life-saving medicines.
At Polymodels Hub, it’s our mission to help pharma companies harness the power of AI and data in standardized workflows to eliminate bottlenecks in R&D.
What if you could plug and play a workflow like ARA? The Automated Risk Assessment tool is just one of many specialized workflows available in ModelFlow. Contact us to learn more.
References
R. Hu et al., ‘Large language model driven transferable key information extraction mechanism for nonstandardized tables’, Sci. Rep., vol. 15, no. 1, p. 29802, Aug. 2025, doi: 10.1038/s41598-025-15627-z.